Novel Cuproptosis-Inhibitory Triketone-sesquiterpene Conjugates Corymbinols A and B from the Fruits of Corymbia citriodora
Corymbinols A (1) and B (2a/2b), novel bis-β-triketone hybrids fused with sesquiterpene and chalcone units, were isolated from Corymbia citriodora fruits. Corymbinols A 1 inhibited cuproptosis in HepG2 cells by 31% at the concentration of 20 μM, implying the potential as a non-toxic therapeutic against liver vancer.
Cuproptosis, a recently identified copper-dependent cell death mechanism, is closely associated with neurodegenerative diseases such as Alzheimer’s and Huntington’s. While copper chelators can mitigate cuproptosis, the development of specific inhibitors remains challenging. In a breakthrough study, a collaborative research team isolated and characterized two unprecedented bis-β-triketone hybrids—Corymbinols A and B —from the fruits of Corymbia citriodora.
Using NMR, electronic circular dichroism (ECD), and X-ray crystallography, the team elucidated the complex structures of these compounds. Corymbinol A features a novel 6/6/10/6/6 pentacyclic scaffold formed via hetero-Diels–Alder (HDA) cycloaddition between hedycaryol (a sesquiterpene) and dual β-triketone units. Corymbinol B comprises enantiomeric bis-β-triketone-dihydrochalcone conjugates.
In a HepG2 cell model induced by elesclomol (ES)/CuCl₂, Corymbinol A demonstrated moderate cuproptosis inhibition at 20 μM, partially reversing copper accumulation-induced cell death. It also restored expression levels of mitochondrial iron-sulfur cluster proteins (ACO2 and SDHB), key markers of cuproptosis. Mechanistic studies suggest that Corymbinol A disrupts copper-mediated protein toxicity without acting as a copper chelator, offering a unique therapeutic strategy.
The study further proposed a biosynthetic pathway for these compounds, starting from β-triketone monomers in Myrtaceae plants. This work not only expands the chemical diversity of phloroglucinol derivatives but also highlights their potential in targeting copper-driven pathologies.
The related research findings, titled “Corymbinols A and B: Bis-β-triketone Conjugates of Sesquiterpene and Chalcone from the Fruit of Corymbia citriodora,” were published in the international journal Organic Letters. Dr. LI Yulin (postdoctoral fellow at South China National Botanical Garden, Chinese Academy of Sciences, SCBG), Associate Researcher DING Xiao (Kunming Institute of Botany, Chinese Academy of Sciences, KIB), and Master’s student DUAN Xianle are co-first authors of the article. Prof. QIU Shengxiang (SCBG) and Prof. and Academician HAO Xiaojiang (KIB) served as co-corresponding authors. This work was supported by the National Natural Science Foundation of China and the Chinese Academy of Sciences Science and Technology Service Network Program. Paper Link: https://doi.org/10.1021/acs.orglett.5c01245
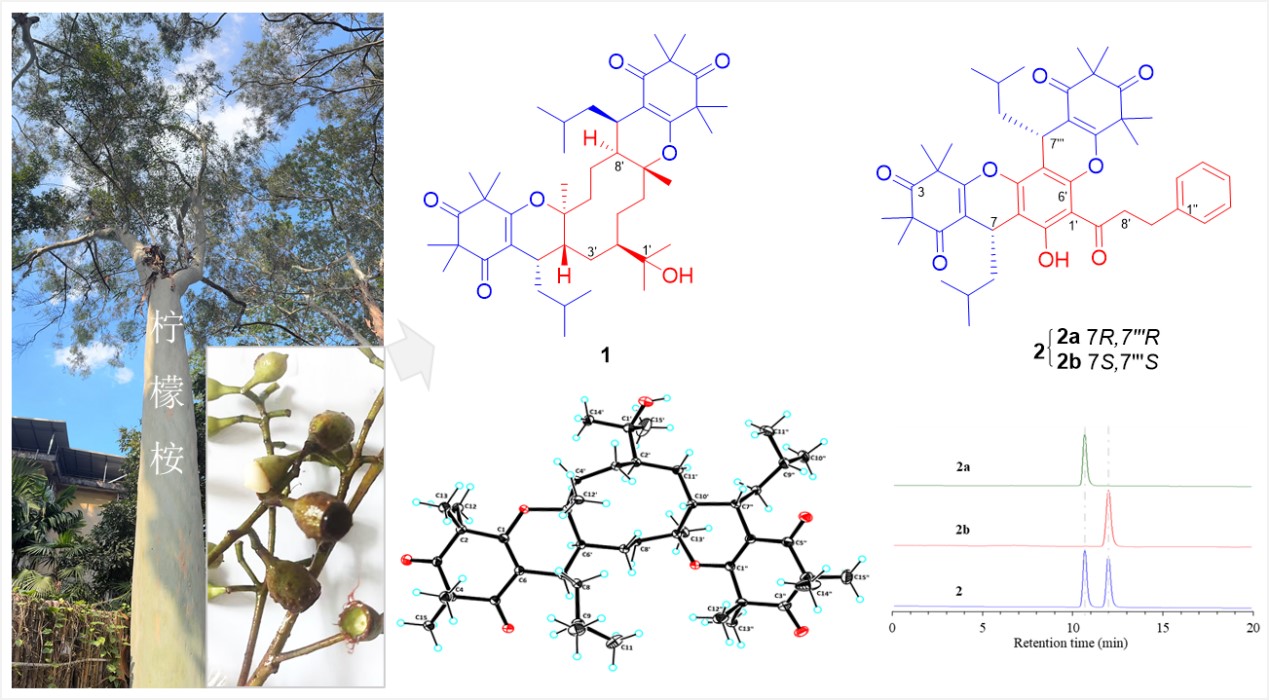
Fig. 1. Novel skeletal structures of Corymbinols A and B isolated from C. citriodora fruits.(Imaged by QIU et al)
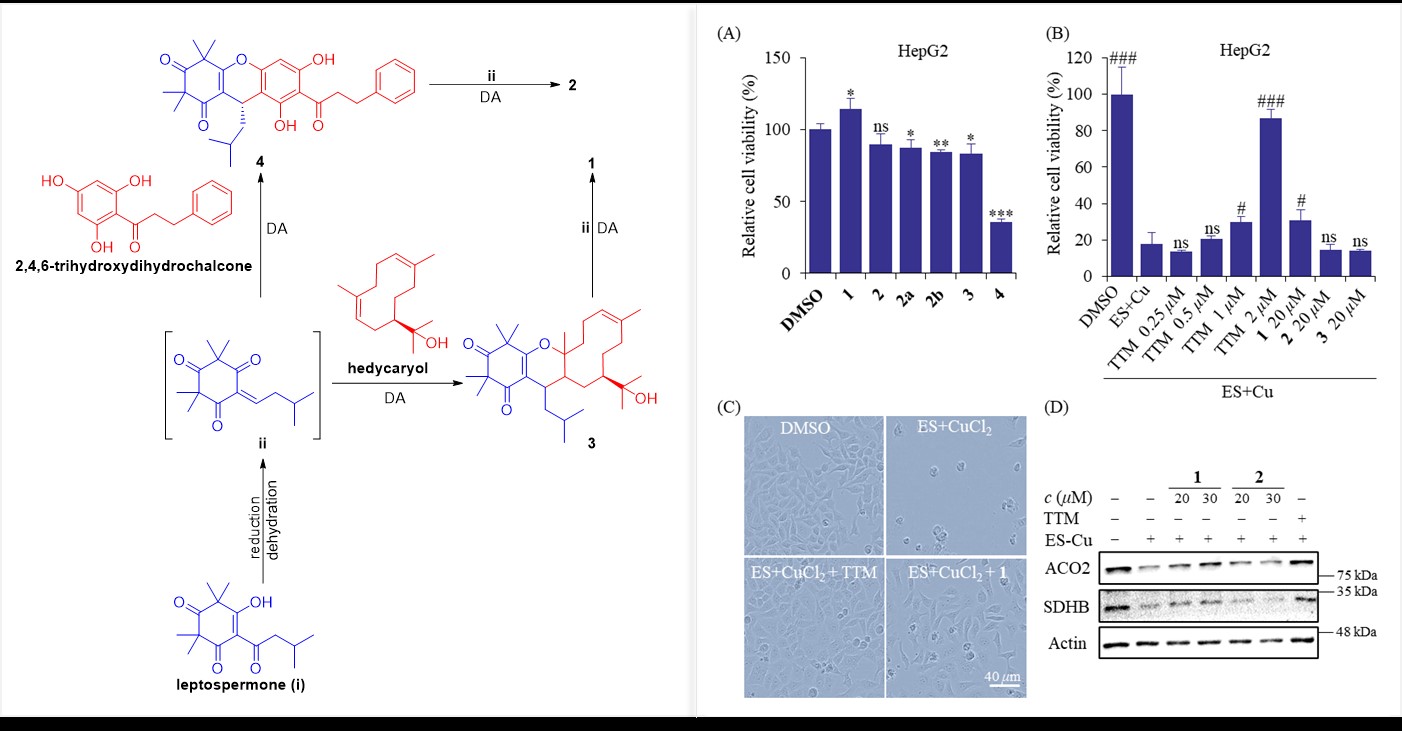
Fig. 2. Proposed biosynthetic pathway (left) and cuproptosis-inhibitory activity of Corymbinol A (right).(Imaged by QIU et al)
File Download: